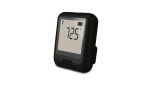The EL-WiFi-21CFR-ULT+ is a 21CFR Part 11-compliant, Cloud-enabled, high-accuracy wireless ultra-low temperature data logger, with a built-in display, designed for monitoring vaccines in ultra-low temperature dry ice storage. The measurement range is -200 to +100°C (-328 to +212°F) using the probe supplied with the logger. The logger automatically records data and uploads it to the EasyLog 21CFR Cloud using your standard wireless network. The device will store data internally if it loses WiFi connection and automatically uploads it to the Cloud once reconnected.
Why buy a 21CFR logger?
The 21 CFR Part 11 regulations issued by the US Food and Drug Administration (FDA) give the criteria under which electronic records can be considered by the FDA as equivalent to signed paper records. If you need to meet these regulations or have enhanced data security requirements then you should choose one of our 21CFR loggers, otherwise our standard loggers will suffice. Learn more about 21 CFR Part 11.
A 21CFR Cloud subscription delivers all of the flexibility of remote monitoring and can be installed as part of a 21CFR-compliant system. Features include permission-based use, authority level sign-off, and full system audit to ensure data monitored and collected is regulated to 21CFR Part 11 standard. This device requires a subscription to our EasyLog 21CFR Cloud service.
This product complies with BS EN 12830:2018 (Temperature recorders for the transport, storage, and distribution of temperature-sensitive goods).
[products skus="EL-P-TC-T-ULT+,PSU USB-UK,PSU USB-EU,EL-WiFi Wall Bracket"]
Calibration testing carried out on our products provides assurance of the ongoing accuracy of your EasyLog data logger. This is relevant for all users, but is especially important for medical, food and scientific applications, and where audit compliance needs to be demonstrated. We provide a number of standard calibration options suitable for common applications such as monitoring fridges, chilled goods and freezers, but can also provide customised calibration at whatever measurement points you need. Please contact our sales team for details.
Calibration options available for the EL-WiFi-21CFR-ULT+:
The EL-WiFi-21CFR-ULT wireless data logger is designed for monitoring vaccines in cryogenic dry ice storage and transit, in accordance with 21CFR Part 11 compliant data security procedures.
For application stories on our data loggers, visit our case studies section.
Vaccine Storage
Whether you’re optimizing processes, safeguarding stock, or storing and transporting valuable assets, reliable data is critical to success.
Pharmaceutical Cold Chain
Using precise temperature loggers in pharmaceutical cold chains to ensure drug efficacy, quality, and health standard compliance.
21CFR Compliant Data Loggers
Offering 21CFR compliant data loggers ensuring secure, reliable data management and regulatory compliance.
21CFR Part 11 is an American FDA regulation covering electronic data records for food and pharmaceutical manufacturers. 21CFR-compliant systems require our 21CFR designated hardware and our separate 21CFR cloud service.
The product has been designed for companies which want to use electronic means to securely replace their paper records to comply with 21 CFR Part 11 regulations for the Pharmaceutical & Medical industries. Also if you work within, and want to be compatible with, the US Pharmaceutical & Medical industry and to streamline your record taking accordingly, initiating a 21 CFR Cloud would be advantageous.
More Support